INTEGRA 9510 Barcode Verifier
The 9510 is extremely simple and intuitive to use and available in four sizes with fields of view of up to 159mm wide. The high resolution 5mp pixel integrated camera allows the verification of very small codes down to an X dimension of 4.0mils (0.10mm) for 1D barcodes and 5.9mils (0.15mm) for 2D codes.
The supplied software is able to auto-discriminate the symbology, narrow bar width and aperture to be used and is 21 CFR Part 11 compliant ready so ideal for use in the pharmaceutical industry.

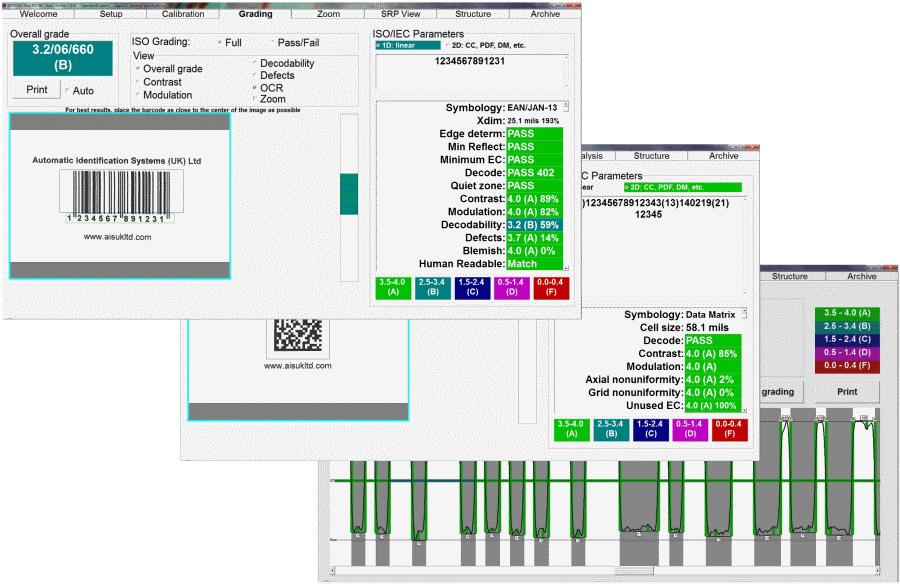
- The INTEGRA 9510 provides full ISO/ANSI barcode verification of 1D and 2D codes.
- All nine ISO/ANSI parameters are verified plus added features of determining blemishes, opacity, and human readable validation.
- Analysis is colour coded to show exactly where a problem is located within the barcode and sections of the code can be analysed to determine how to overcome it.
- High-resolution inspection of the barcode analyses every 0.05mm of the barcode height which exceeds the minimum ten scan average required by ISO standards.
- Multiple codes including any combination of Linear, 2D and Stacked Linear barcodes can be verified on one label within the field of view.
- All 1D and 2D barcode application standards are supported including ISO 15415, ISO 15416, GS1 General Specifications, Mailmark, HIBC, MIL-STD-130N, French CIP, AIG/ODETTE and many more.
- Accuracy and repeatability are paramount in barcode verification. The operator is not involved in the scanning process with no wand to hold, no angle to maintain and no buttons to press.
- The INTEGRA 9510 is the most reliable system on the market. There are no moving parts to wear and no laser diode to burn out.
- Every system is supplied with a NIST traceable conformance standard test card provided by GS1 to ensure that it is always within a known calibration standard.
- The INTEGRA 9510 is designed to grade barcodes to ISO standards on various label sizes and finished products with flat or rounded sides.
- The INTEGRA 9510 is certified by GS1 US and is 21 CFR Part 11 compliant ready making it ideal for use in pharmaceutical and medical device industries.

-med.jpg)